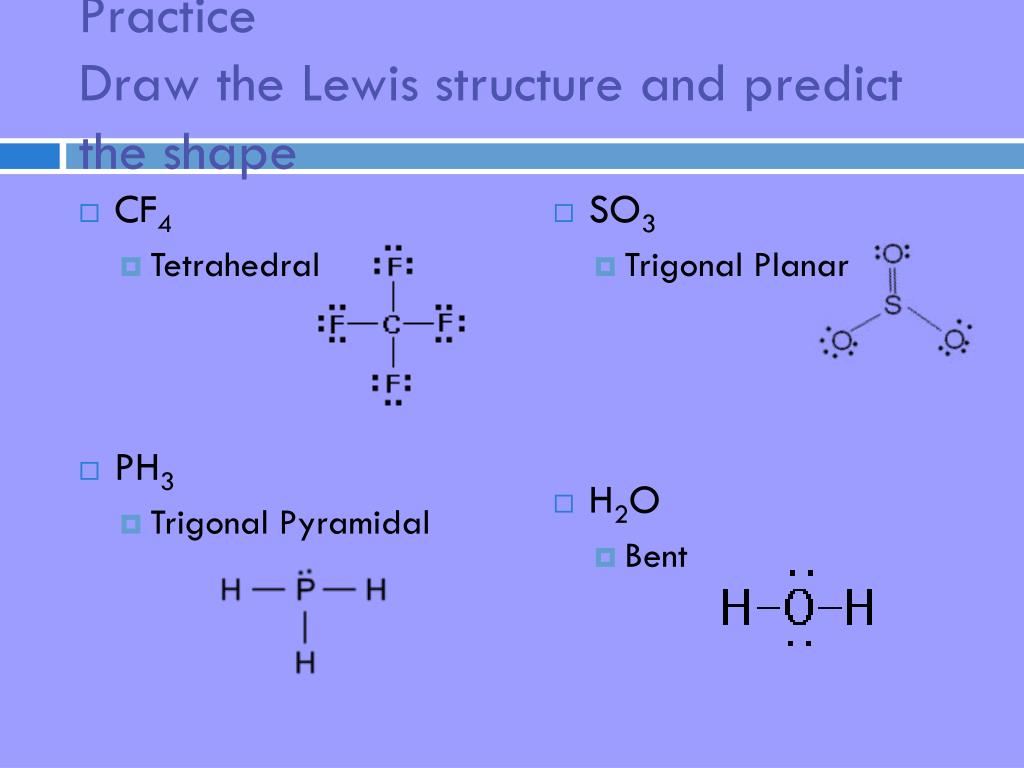
Ph3 Lewis Structure Shape
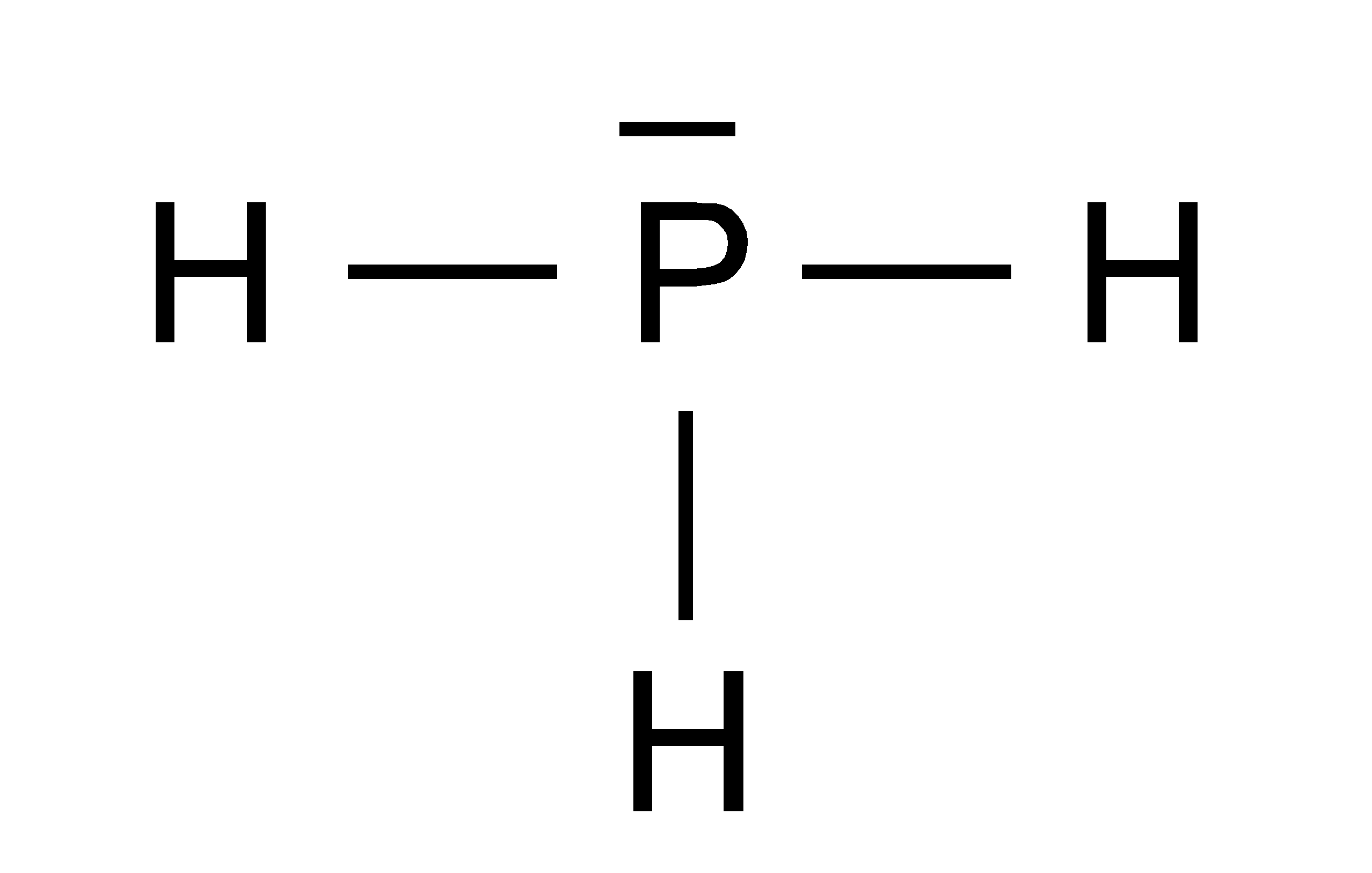
PH3 lewis structure has a Phosphorus atom (P) at the center which is surrounded by three Hydrogen atoms (H). There are 3 single bonds between the Phosphorus atom (P) and each Hydrogen atom (H). There is 1 lone pair on the Phosphorus atom (P).
Ph3 Lewis Structure Shape
The Lewis structure for PH 3 is similar to NH 3. In the PH 3 Lewis structure (and all Lewis structures) hydrogen goes on the outside. Remember, too, that hydrogen only needs two valence electrons to have a full outer shell. In the Lewis structure for PH 3 there are a total of 8 valence electrons. Three pairs will be used in the chemical bonds.
Ph3 Lewis Structure Shape
From the Lewis molecular structure of PH3, we have seen the phosphorous atom has five valence electrons. During the bonding process, Phosphorous is surrounded by three hydrogen atoms, and each is connected by a single bond. The two remaining electrons form a lone pair.
PPT Covalent Bonds PowerPoint Presentation, free download ID3048466
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

Lewis dot structure of PH3 (phosphine)... YouTube
Step 1 (A) Lewis structure of PH A 3 View the full answer Step 2 Unlock Answer Unlock Previous question Next question Transcribed image text: Part A Draw the Lewis structure of PH3 To add lone pairs, click the button before clicking on the molecule.

How to draw PH3 Lewis Structure? 4
How do you draw the lewis structure for ph3? Chemistry 1 Answer Ernest Z. · Christopher P. Jan 5, 2014 Here are the steps that I follow when drawing a Lewis structure. Decide which atom is the central atom in the structure. That will normally be the least electronegative atom (P).

Draw The Lewis Structure Of Ph3
PH3 or phosphine is a compound of phosphorus that is classified under pnictogen hydride. Phosphine or phosphane forms bonds by covalent bonding. We can study the bonding in the molecule of PH3 by taking into consideration lewis method. We will study the PH3 lewis structure and understand the concept. Some facts about Phosphane
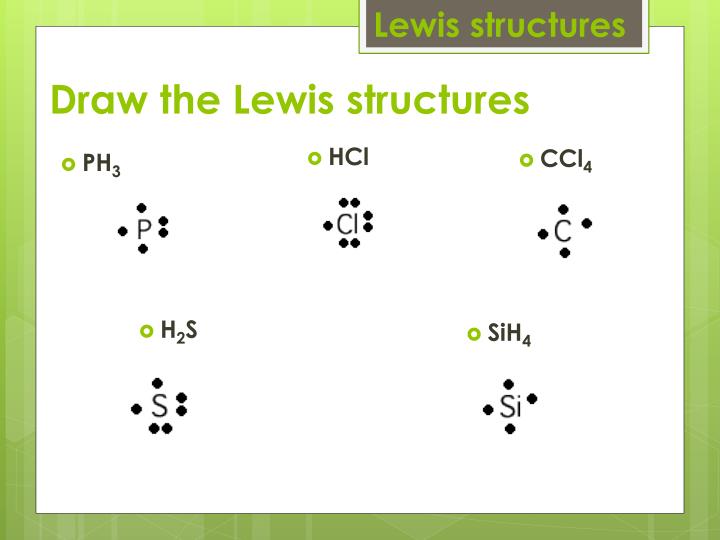
11+ Ph3 Lewis Structure Robhosking Diagram
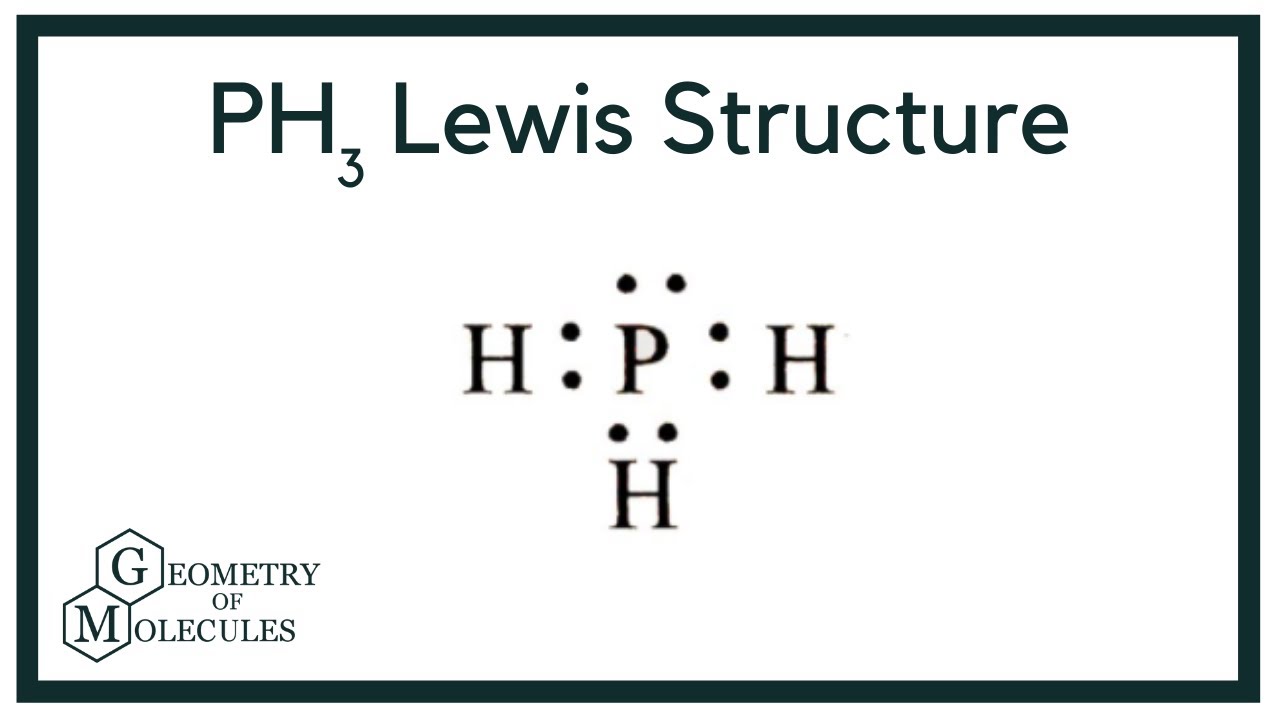
In the PH 3 Lewis structure, there are three single bonds around the phosphorus atom, with three hydrogen atoms attached to it, and on the phosphorus atom, there is one lone pair. PH3 Lewis Structure - How to Draw the Lewis Structure for PH3 Watch on Contents Steps #1 Draw a rough sketch of the structure #2 Next, indicate lone pairs on the atoms

Step2 Lewis Structure of PH3 for constructing around the central
This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
Ph3 Lewis Structure Shape
This tutorial shows you how to create the Lewis structure and moleculargeometry for phosphine (PH3).

Ph3 Lewis Structure Shape
Lewis Structures for PH3. Step-by-step tutorial for drawing the Lewis Structure for PH3.

Intermolecular Forces for PH3 YouTube
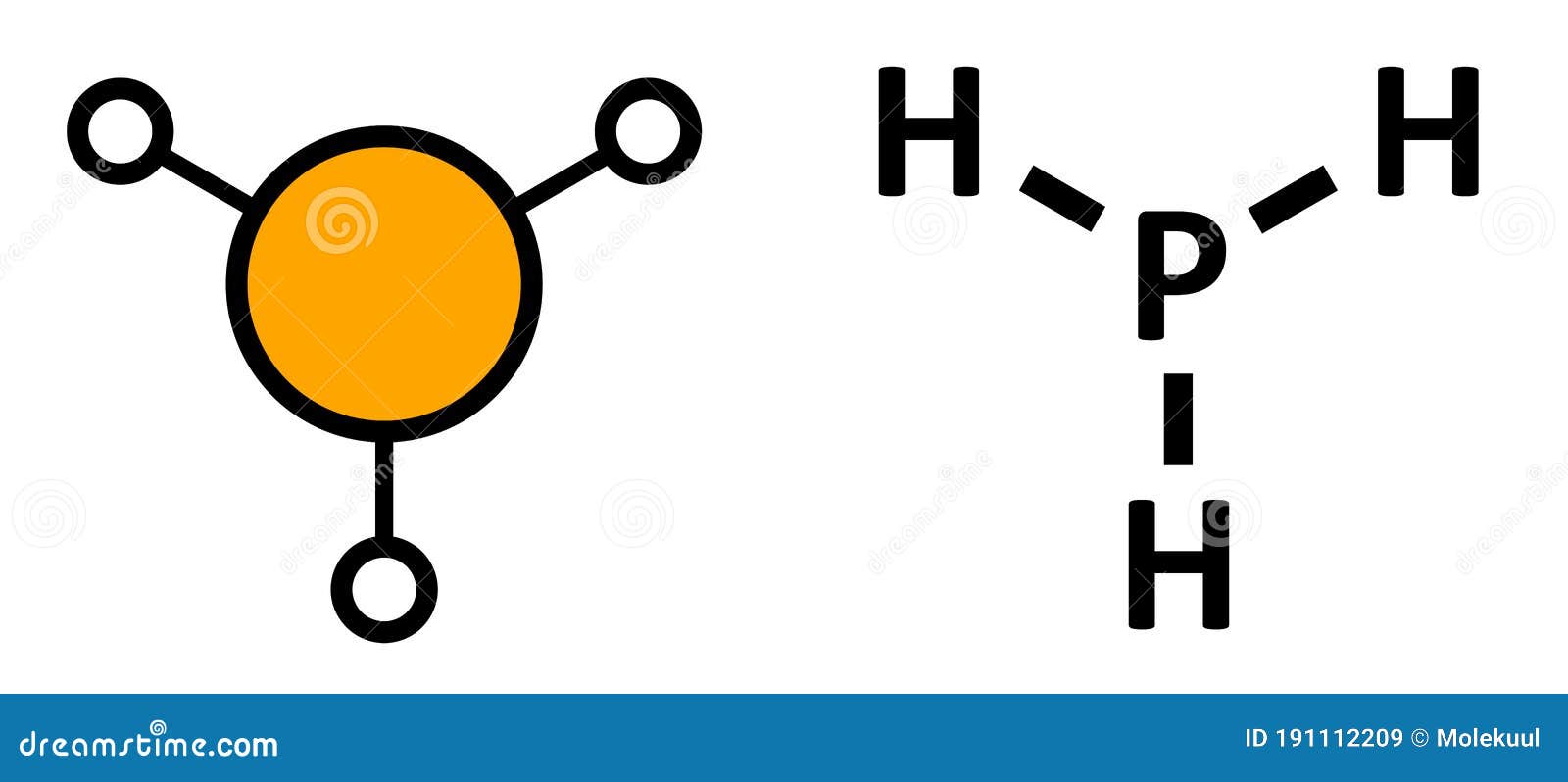
In phosphine (PH 3) lewis structure, there are three sigma bonds and one lone-pair around phosphorous atom. No charges on phosphorous atom and hydrogen atoms. Shape of PH 3 is trigonal pyramidal. Molecular geometry around phosphorous atom is tetrahedral. Total valence electrons pairs around phosphorous atom is four.

PH3 Lewis Structure How to Draw the Lewis Structure for PH3 YouTube
The Lewis electron structure for the NH 4+ ion is as follows: The nitrogen atom shares four bonding pairs of electrons, and a neutral nitrogen atom has five valence electrons. Using Equation 4.4.1, the formal charge on the nitrogen atom is therefore. formalcharge (N)=5− (0+82)=0 .
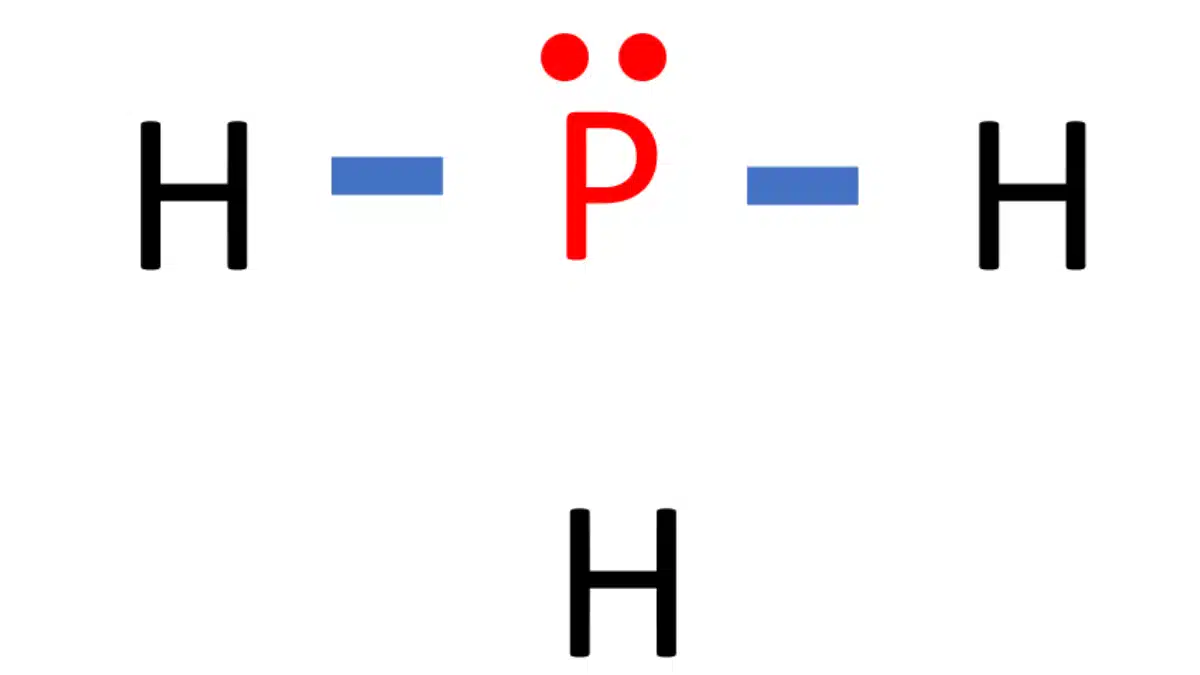
PH3 Lewis Structure in four simple steps What's Insight
Lewis structure of PH3 contains three single bonds between the Phosphorus (P) atom and each Hydrogen (H) atom. The Phosphorus atom (P) is at the center and it is surrounded by 3 Hydrogen atoms (H). The Phosphorus atom has one lone pair. Let's draw and understand this lewis dot structure step by step.

Ph3 Lewis Structure Shape
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of.
PH3 Lewis Structure (Phosphine) YouTube
Lewis Structure is the pictorial representation of the arrangement of atoms and valence electrons in the molecule. To know the Lewis Structure, we first know the central atom and the arrangement of other atoms. Here for PH3, the phosphorus atom will take the central position as Hydrogen atoms cannot take a central position in the Lewis Structure.